- Sileo must not be used in dogs with sev ere disorders of the heart and blood vessels, with severe disease such as kidney or liver failure or in dogs if unexpectedly sedated from a previous dose. For a full list of all restrictions and side -effects reported with Sileo, see the package leaflet.
- DEXDOMITOR is also indicated for use as a preanesthetic to general anesthesia in dogs. Dosage and Administration. Dogs: DEXDOMITOR produces sedation and analgesia when administered intramuscularly (IM) at a dose of 500 mcg/m 2, or intravenously (IV) at a dose of 375 mcg/m 2. Doses for preanesthesia are 125 or 375 mcg/m 2 IM.
- Sileo Dose In Cats Treatment Sileo For Dogs Side Effects The first dose should be given as soon as the dog shows the first signs of anxiety, or when the owner detects a typical stimulus (e.g. Sound of fireworks or thunder) for eliciting anxiety or fear in the respective dog.
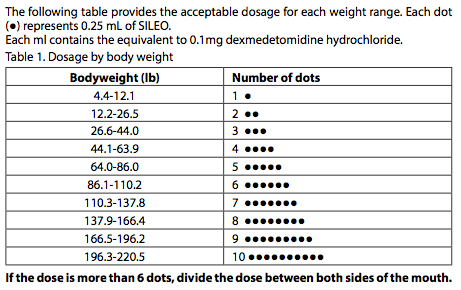
- Sileo® 0.1 mg/ml oromucosal gel for dogs. Sileo® 0.1 mg/ml oromucosal gel for dogs. Zoetis UK Limited. (in dots) to be administered for the corresponding bodyweight. If the dose for the dog is more than 6 dots (1.5 ml), half of the dose should be administered to the oral mucosa on one side of the dog’s mouth and the other.
Hypertension in Cats
Because Sileo is formulated to provide a low dose of dexmedetomidine, your dog remains calm, yet fully functional, and is able to interact normally with your family. SILEO is indicated for the treatment of noise aversion in dogs.
A silent disease
While hypertension can be a common problem in older cats, the condition can be difficult to diagnose.3,4
Hypertension can affect many parts of the body before detection and is strongly associated with target organ damage3,4:
- Eyes
- Heart and vascular system
- Brain
- Kidneys
Without treatment, this silent disease can lead to serious illness and, sometimes, death.3,4
Most often develops as secondary hypertension to diseases such as CKD and hyperthyroidism.3
Efficacy
Proven to lower systolic blood pressure1,10
A pilot dose determination laboratory study was conducted to evaluate the effectiveness of multiple oral doses and dose strategies of telmisartan on the reduction of systolic blood pressure (SBP) in clinically normal, healthy laboratory cats within a 14-day time period.11
* Endpoint at day 14 was significant reduction from baseline compared to placebo (P < 0.0005).
† A clinically relevant reduction in group mean SBP was predefined to be ≥ 20 mmHg in the telmisartan group from baseline to Day 28. The number of cats available at Day 28 for comparison is less than at enrollment because of removal of cats for hypertension rescue or adverse reactions.
The only angiotensin receptor blocker (ARB) for first-line treatment of hypertension in cats
Because ARBs feature a unique, targeted mode of action that specifically blocks the harmful vasoconstrictive effects of the renin-angiotensin-aldosterone system (RAAS), SEMINTRA is shown to quickly lower blood pressure within 14 days‡
- Shown to be effective in cats with primary and secondary hypertension
- Proven to be safe and effective10–13
Safety and Dosing
Flexible dosing to safely lower and maintain blood pressure in cats
- SEMINTRA offers a dose-dependent response—an initial 1.5 mg/kg (0.68 mg/lb) dose twice daily for 14 days lowers blood pressure to a healthy level quickly, followed by a 2 mg/kg (0.91 mg/lb) once daily dose to maintain control11
- Dose may be reduced by 0.5 mg/kg (0.23 mg/lb) increments to a minimum of 0.5 mg/kg to optimize dosing and prevent hypotensive state
Easily given as oral liquid
Easy-to-use Syringe
— Flexible delivery of dose straight into the mouth or on top of food2
— Selection of accurate dose
Safe for long-term administration
— Safety shown to extend through 6 months in multiple studies10–13

Convenient liquid formulation
As demonstrated in a study:
— 91.2% of cat owners perfer a liquid formulation2
Mode of Action

SEMINTRA is a highly targeted treatment

Telmisartan, the active substance in SEMINTRA, is an ARB, a class of medication that modulates the RAAS by selectively blocking receptors in the effector step of the RAAS. Unlike angiotensin-converting enzyme inhibitors (ACEis), ARBs do not block the conversion of angiotensin I to angiotensin II by angiotensin-converting enzyme in the RAAS cascade, thereby also blocking the activation of some of the receptor types with beneficial effects. And, unlike a calcium channel blocker, a class of pharmaceuticals that block the influx of calcium into cardiac and smooth muscle cells through calcium channels and potentially activate the RAAS, ARBs help to manage the RAAS.
Chronic activation of the RAAS can be harmful to tissues, including the kidney.
Sileo Dog Dose
Watch this video for more information
- Activation of RAAS can be complex, but conditions like chronic kidney disease, cardiac disease, and hyperthyroidism typically increase RAAS activity
- Chronic, excessive RAAS activation can result in systemic hypertension because it promotes salt and water retention and increases blood pressure14,15
- ACEis reduce the conversion of circulating angiotensin I to angiotensin II, which reduces all of the effects of angiotensin II at all receptors
- ARBs have a more targeted mode of action. They bind only to the AT1 receptor, preventing activation of this receptor by angiotensin II
- Because ARBs do not bind to AT2 receptors, the potentially beneficial effects of angiotensin II at these receptors are preserved
Sileo Dose In Cats Treatment
Watch this video for more information
Target-specific mode of action of ARBs
IMPORTANT SAFETY INFORMATION
- SEMINTRA is for oral use in cats only.
- The most common side effects reported in field studies include vomiting, diarrhea, lethargy, dehydration, poor appetite, and weight loss. If side effects should occur, pet owners should contact their veterinarian. Click here for full prescribing information.
- SEMINTRA has not been evaluated in cats with systolic blood pressure > 200 mmHg. The safe use of SEMINTRA has not been evaluated in cats with hepatic disease, cats less than 9 months of age, or cats that are pregnant, lactating, or intended for breeding.
- The safe use of SEMINTRA with other anti-hypertensive medications has not been evaluated.
- Mild anemia or non-regenerative anemia has been reported.
- Pregnant women should avoid contact with SEMINTRA because substances that act on the renin-angiotensin-aldosterone system (RAAS), such as angiotensin receptor blockers (ARBs), can cause fetal and neonatal morbidity and death during pregnancy in humans.
- Not for human use. Keep out of reach of children. If SEMINTRA is accidentally ingested, contact a physician.
- SEMINTRA is a clear, colorless to yellowish viscous solution containing 10 mg/mL telmisartan.
References:1. SEMINTRA® (telmisartan oral solution) Prescribing Information. Boehringer Ingelheim Vetmedica, Inc. 2018. 2. Zimmering T. Ease of use of SEMINTRA and its effects on quality of life—update on cat owner feedback (“EASY Programme”) [abstract]. In: Proceedings from the 21st Federation of European Companion Animal Veterinary Associations (FECAVA); October 15–17, 2015; Barcelona, Spain. Poster. 3. Elliott J, Fletcher M, Syme HM. Idiopathic feline hypertension: epidemiologic study [abstract]. J Vet Intern Med. 2003;17:754. 4. Taylor SS, Sparkes AH, Briscoe K, et al. ISFM Consensus Guidelines on the diagnosis and management of hypertension in cats. J Feline Med Surg. 2017;19(3):288–303. 5. Maggio F, DeFrancesco TC, Atkins CE, et al. Ocular lesions associated with systemic hypertension in cats: 69 cases (1985–1998). J Am Vet Med Assoc. 2000;217:695–702. 6. Littman MP. Spontaneous systemic hypertension in 24 cats. J Vet Intern Med. 1994;8:79–86. 7. Stiles J, Polzin D, Bistner SI. The prevalence of retinopathy in cats with systemic hypertensionand chronic renal failure or hyperthyroidism. J Am Anim Hosp Assoc. 1994;30:564–572. 8. Syme HM, Barber PJ, Markwell PJ, et al. Prevalence of systolic hypertension in cats with chronic renal failure at initial evaluation. J Am Vet Med Assoc. 2002;220:1799–1804. 9. Kobayashi DL, Peterson ME, Graves TK, et al. Hypertension in cats with chronic renal failure orhyperthyroidism. J Vet Intern Med. 1990;4:58–62. 10. Coleman AE. Efficacy of oral telmisartan for the treatment of systemic hypertension in cats. In: Proceedings from the American College of Veterinary Internal Medicine; June 14–16, 2018; Seattle, WA. Abstract RRS08-E. 11. Coleman AE, Brown SA, Stark M. Evaluation of orally administered telmisartan for the reduction of indirect systolic arterial blood pressure in awake, clinically normal cats [published online ahead of print March 7, 2018]. 12. Glaus AM, Elliott J, Albrecht B, et al. Efficacy of telmisartan in hypertensive cats: results of a large European clinical trial [abstract]. J Vet Intern Med. 2018;32:577. 13. SEMINTRA® (telmisartan oral solution) [Freedom of Information Summary]. St. Joseph, MO: Boehringer Ingelheim Vetmedica, Inc.; 2018. J Feline Med Surg. doi:10.1177/1098612X18761439. 14. Williams TL, Elliott J, Syme HM. Renin-angiotensin-aldosterone system activity in hyperthyroid cats with and without concurrent hypertension. J Vet Intern Med. 2013;27(3):522–529. 15. Syme H. Hypertension in small animal kidney disease. Vet Clin North Am Small Anim Pract. 2011;41(1):63–89.